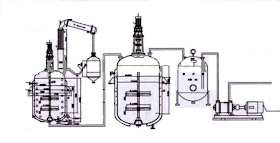
Process description
Alkyd resin can be manufactured by four different processes, solvent process being the main commercial process which starts by adding all the raw materials in reaction kettle and the agitation of the same is continued then, inert gas is sparged through the spare line. The vapour are condensed and recycled back to the kettle.
The reacted mass is dropped into a jacketed tank, containing the desired solvent. The cooled resin solution after thinning is filtered in filter press and sludge is removed. It is lastly tested,
packed and stored.
packed and stored.
The solvent process uses a small amount of solvent, 5-10%, in the esterfication reaction to act as a reflux medium.
The advantages of this process are:
a) Uniformity of product,
b) Increased speed of reaction and
c) Lower material losses.
In the solvent process, the production of alkyds can be carried out either in a single stage or a two stage process. Under the single stage process, the drying oil (linseed oil), polyalcohol and phthalic anhydride are converted simultaneously. This method of alkyd preparation is not satisfactory because of the incompatibility of the phthalic anhydride with drying oil (linseed oil) and the difficulty of controlling the reaction to produce the desired end-products.
In the first sage of the two stage solvent process, monoglyceride is produced from drying oil and polyalcohol and in the second stage the monoglyceride is converted with phthalic anhydride. This process is more satisfactory and is the one recommended for the envisaged plant because it eliminates the problems of the first option.
In the two- stage solvent process, the first operation is the alcholoysis reaction which takes place under different duration of time (varying form 40 minutes to 4 hours) and temperature (from about 240 to 260oC). The completion of this stage is shown by the solubility of the product in about twice its weight of methanol.
The second stage which takes place in the same reactor is the actual formation of the alkyd proper. The monoester formed in the first stage is cooled slightly to about 210oC and the requisite quantity of phthalic anhydride is added. The resin is then partially cooled with water coils, and at the same time a thinning solvent is added. The partially diluted alkyd solution is run into a blender, passing through a filter press to remove any gel particles that are formed. The completed alkyd solution is then pumped to a second filter press for clarification and then to storage tanks or immediate use.
Synthesis from Oils or Fatty Acids.
Monoglyceride Process. In the case of glycerol alkyds, it would be absurd to first saponify an oil to obtain fatty acids and glycerol, and then reesterify the same groups in a different combination. Rather, the oil is first reacted with sufficient glycerol to give the total desired glycerol content, including the glycerol in the oil. Since PA is not soluble in the oil, but is soluble in the glycerol, transesterification of oil with glycerol must be carried out as a separate step before the PA is added; otherwise, glyceryl phthalate gel particles would form early in the process. This two-stage procedure is often called the monoglyceride process.
The transesterification reaction is run at 230–250◦C in the presence of a catalyst; many catalysts have been used. Before the strict regulation of lead in coatings, litharge (PbO)was widely used; the residual transesterification catalyst also acted as a drier. Examples of catalysts now used in the United States are tetraisopropyl titanate, lithium hydroxide, and lithium ricinoleate. The reaction is run under an inert atmosphere such as CO2 or N2 to minimize discoloration and dimerization of drying oils. Rather than using glycerol, the transesterification can also be carried out with higher functionality polyols such as pentaerythritol.
Fatty Acid Process. It is often desirable to base an alkyd on a polyol (eg, pentaerythritol) other than glycerol. In this case, fatty acids must be used instead of oils, and the process can be performed in a single step with reduced time in the reactor. Any drying, semidrying, or nondrying oil can be saponified to yield fatty acids, but the cost of separating fatty acids from the reaction mixture increases the cost of the alkyd. A more economical alternative is to use TOFA, which have the advantage that they are produced as fatty acids. Tall oil fatty acid composition is fairly similar to that of soybean fatty acids. Specially refined tall oils with higher linoleic acid content are available, as are other grades that have been treated with alkaline catalysts to isomerize the double bonds partially to conjugated structures. Generally, when fatty acids are used, the polyol, fatty acids, and dibasic acid are all added at the start of the reaction, and the esterification of both aliphatic and aromatic acids is carried out simultaneously in the range of 220–255◦C.
“Alkyd Resins” in EPST 1st ed., Vol. 1, pp. 663–734, by R. G. Mraz and R. P. Silver, Hercules
Powder Co.; “Alkyd Resins” in EPSE 2nd ed., Vol. 1, pp. 644–679, by H. J. Lanson, Lan Chem
Corp.
Related posts
Synthesis from Oils or Fatty Acids.
Monoglyceride Process. In the case of glycerol alkyds, it would be absurd to first saponify an oil to obtain fatty acids and glycerol, and then reesterify the same groups in a different combination. Rather, the oil is first reacted with sufficient glycerol to give the total desired glycerol content, including the glycerol in the oil. Since PA is not soluble in the oil, but is soluble in the glycerol, transesterification of oil with glycerol must be carried out as a separate step before the PA is added; otherwise, glyceryl phthalate gel particles would form early in the process. This two-stage procedure is often called the monoglyceride process.
The transesterification reaction is run at 230–250◦C in the presence of a catalyst; many catalysts have been used. Before the strict regulation of lead in coatings, litharge (PbO)was widely used; the residual transesterification catalyst also acted as a drier. Examples of catalysts now used in the United States are tetraisopropyl titanate, lithium hydroxide, and lithium ricinoleate. The reaction is run under an inert atmosphere such as CO2 or N2 to minimize discoloration and dimerization of drying oils. Rather than using glycerol, the transesterification can also be carried out with higher functionality polyols such as pentaerythritol.
Fatty Acid Process. It is often desirable to base an alkyd on a polyol (eg, pentaerythritol) other than glycerol. In this case, fatty acids must be used instead of oils, and the process can be performed in a single step with reduced time in the reactor. Any drying, semidrying, or nondrying oil can be saponified to yield fatty acids, but the cost of separating fatty acids from the reaction mixture increases the cost of the alkyd. A more economical alternative is to use TOFA, which have the advantage that they are produced as fatty acids. Tall oil fatty acid composition is fairly similar to that of soybean fatty acids. Specially refined tall oils with higher linoleic acid content are available, as are other grades that have been treated with alkaline catalysts to isomerize the double bonds partially to conjugated structures. Generally, when fatty acids are used, the polyol, fatty acids, and dibasic acid are all added at the start of the reaction, and the esterification of both aliphatic and aromatic acids is carried out simultaneously in the range of 220–255◦C.
“Alkyd Resins” in EPST 1st ed., Vol. 1, pp. 663–734, by R. G. Mraz and R. P. Silver, Hercules
Powder Co.; “Alkyd Resins” in EPSE 2nd ed., Vol. 1, pp. 644–679, by H. J. Lanson, Lan Chem
Corp.
Related posts