Although the fast pyrolysis process is more accurately called thermolysis, the two terms are used interchangeably. Since 'pyro' means fire in Greek and 'thermo' means heat, thermolysis more closely describes the process, which involves heating biomass through indirect heat. The term 'fast' refers to the residence time of the biomass in the reactor. Slow pyrolysis produces more solid char, while fast pyrolysis yields more gas and liquid. The reactor design, temperature, and residence time determine the yields of the products and to some extent, their qualities.
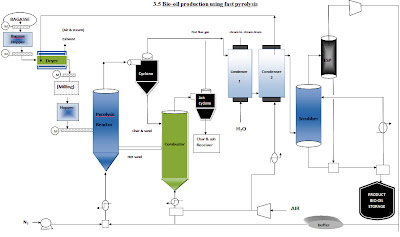
In the fast pyrolysis process, indirect heat, followed by cooling, is used to convert biomass at 400-800°C into CO2, CO, and CH4 gases (medium heating value gases), Bio- Oil liquids and solid char. A flow diagram of the process is shown in figure below. The conversion of biomass to Bio-Oil occurs in the absence of oxygen in the pyrolysis reactor . Oxygen is excluded to prevent the biomass or its products from combustion.(use a N2 gas as a fluidizing medium) Gasification is different from fast pyrolysis, or thermolysis, because it is a combustion process in which enough oxygen, through air, is supplied for the carbon and hydrogen in the fuel to form a rich mixture of CO and hydrogen in the product gas. The product gas from gasification will contain up to 60% nitrogen and have a lower heating value of 107 to 484 Btu/ft3 whereas the heating value of fast pyrolysis gases range from 484 to 807 Btu/ft3 (www.wgtuk.com/ukDefinitionGas.html).
The products exit the pyrolysis reactor in a gas state that includes vapors, aerosols, and a suspension of small solid particles. A cyclone separates most of the solid particles from the vapors, where they are then deposited to the char collector. The gases are then condensed in a quench system, which converts most of the vapor into liquid Bio-Oil. The non-condensable gases are recycled to supply the energy needed in the process, while the Bio-Oil and char can be sold as commercial products (www.DynaMotive.com/biooil).